This process includes the separation of different dissolved constituents of a mixture by absorbing them over an appropriate material which is capable of being an absorbent is called Chromatography. Usually, the absorbent is,
*Magnesium oxide
*Alumina
*Filter paper
The four main types of Chromatography are,
•Liquid chromatography
•Gas chromatography
•Thin layer chromatography and
• Paper chromatography
Now, let’s understand what paper chromatography is. In analytical chemistry, this is a common technique that is widely used for the separation of Chlorophyll pigments, amino acids, DNA bases, etc.
In this process, the paper acts as a solid material and the water molecules that are present in the paper will act as a stationary phase while the mixture of different solvents can be used as a phase for mobility.
A capillary is used to apply the sample on the paper. After the application of the sample on the paper, the chromatography process can take place in different modes. Talking about modes, there are many types in this also. They can be in Ascending mode, Descending mode, Radial mode, and in the 2-Dimensional model.
Read More: Education is Key to Achieving Sustainability in a Rapidly Changing World
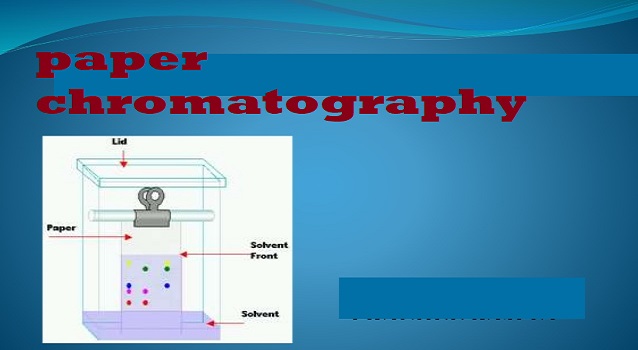
PRINCIPLE OF PAPER CHROMATOGRAPHY
The separation of mixed components using paper as the stationary phase.
An experiment can be conducted to prove or to check its technique. The materials required for this experiment include A paper for the stationary phase, A solvent for the mobile phase, and a sample of your wish.
TECHNIQUE
Firstly, you have to take a filter paper. Then, the paper should be marked with the starting and the ending point. In the next step, the sample has to be placed on the line of the start point. Now the stationary phase has got ready. After this, the stationary phase has to dip into the mobile phase which consists of the solvent. In simple words, the paper should be dipped into the solvent.
We have to keep in mind that the whole of the stationary phase should not be dipped into the solvent, only the part that has the sample on it has to be dipped.
OBSERVATION
After the experiment, you can see that the substances from the sample migrate to the other point marked on the paper in different colors. The migration of the particles depends upon three categories
• Size of the sample
• Nature of the sample
• KD value of the sample
This result can be observed in five-ten minutes.
Now a question might arise to you. “What is the purpose of paper chromatography?” Or “What is its main purpose?”
It is used to find the RF value.
The formula to find this RF value is, Distance traveled by the solute from the origin divided by the distance traveled by the solvent from the origin.
RF value cannot be found without the experiment.
There are two types of techniques involved.
1 Dimensional technique
2-Dimensional technique
In paper chromatography, the 2-Dimensional technique will be used mostly.
Rarely, dimensional will be used. Proper results can be obtained from the second technique. The first technique might provide wrong results and lead to a mixture of the substances that migrate from one point to another. They may get diffused in the first method.
Difference between one- and two-dimensional technique.
1D – the stationary phase will be presented vertically.
2D – the stationary phase will be presented horizontally.
Things that need to be in check before the development of this chromatography:
1)The paper type
2) Paper preparation
3)Solute mixture preparation
4)Spotting of solute mixture
5) Solvent choosing
6) Technique Development
Later, the paper has to be cut in an appropriate size, shape. Then the paper has to be washed so that the impurities present in it will also be washed away. The sample that is chosen has to be taken in the microgram quantity level not even at the milligram level.
During the solvent selection for the mobile phase. Three types of solvents are used.
•Polar solvents
• Non-polar solvents
•Moderate polar solvents.
They can be chosen according to the sample selection.
Paper Chromatography in real life is used to segregate or separate the different constituents present in a mixture. It is used to find the components present in drugs. Pharmaceutical industries and companies use this technique. Antibiotics can be tested using this method.
Pollutants in the water can be separated using this method. It is also used to detect spoilage in the food that we eat. Paper Chromatography is a type of planar chromatography that can run only on a specialized type or kind of paper.